Peptide Cancer Vaccine Market Poised for Rapid Growth, Set to Hit USD 4.93 Billion by 2034
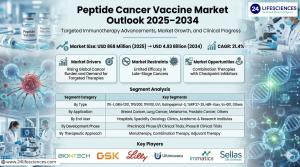
Peptide cancer vaccine market is set to expand from USD 868 million in 2025 to USD 4.93 billion by 2034, registering a strong 21.4% CAGR.
Rising from USD 868 million in 2025 to USD 4.93 billion by 2034, the peptide cancer vaccine market reflects rapid innovation and a robust 21.4% CAGR.”
PUNE, MAHARASHTRA, INDIA, December 24, 2025 /EINPresswire.com/ -- Peptide Cancer Vaccine Market was valued at 𝗨𝗦𝗗 𝟴𝟲𝟴 𝗺𝗶𝗹𝗹𝗶𝗼𝗻 𝗶𝗻 𝟮𝟬𝟮𝟱 and is projected to reach 𝗨𝗦𝗗 𝟰.𝟵𝟯 𝗯𝗶𝗹𝗹𝗶𝗼𝗻 𝗯𝘆 𝟮𝟬𝟯𝟰, growing at a 𝗖𝗔𝗚𝗥 𝗼𝗳 𝟮𝟭.𝟰% during the forecast period. — 2424lifesciences
Peptide Cancer Vaccine Market is steadily emerging as a critical pillar within modern oncology, driven by the growing shift toward personalized and targeted cancer therapies. As researchers and healthcare systems seek treatments that activate the body’s immune system with greater specificity and fewer systemic side effects, peptide-based vaccines are gaining renewed clinical and commercial attention.
Peptide cancer vaccines are especially well-suited for customized treatment plans because, in contrast to traditional cancer treatments, they are intended to elicit tumor-specific immune responses. Ongoing advances in immunology, bioinformatics, and peptide synthesis are further strengthening their therapeutic potential across multiple cancer indications.
💠𝐅𝐞𝐞𝐥 𝐅𝐫𝐞𝐞 𝐭𝐨 𝐑𝐞𝐚𝐜𝐡 𝐎𝐮𝐭 𝐭𝐨 𝐔𝐬 𝐟𝐫𝐨𝐦 𝐓𝐡𝐢𝐬 𝐒𝐚𝐦𝐩𝐥𝐞 𝐋𝐢𝐧𝐤: https://www.24lifesciences.com/download-sample/7842/peptide-cancer-vaccine-market
𝗟𝗮𝘁𝗲𝘀𝘁 𝗨𝗽𝗱𝗮𝘁𝗲𝘀 𝗮𝗻𝗱 𝗜𝗻𝗻𝗼𝘃𝗮𝘁𝗶𝗼𝗻𝘀:
◘ 𝗠𝗮𝗿𝗰𝗵 𝟮𝟬𝟮𝟱: Researchers at the Icahn School of Medicine at Mount Sinai, led by MD, PhD, Ward-Coleman Chair in Cancer Research and Director of the Vaccine and Cell Therapy Laboratory, have tested a promising new type of personalized multi-peptide neoantigen cancer vaccine, called PGV001, in a small group of patients. This early research (phase 1 trial) is crucial to developing more effective cancer treatment strategies. In order to help the body's immune system identify and combat cancer cells and prevent the disease from returning, the vaccine makes use of many peptides, or amino acid sequences.
◘ 𝗔𝘂𝗴𝘂𝘀𝘁 𝟮𝟬𝟮𝟱: Elicio Therapeutics’ lead cancer vaccine asset, ELI-002, has been found to improve survival outcomes in patients with hard-to-treat solid tumors. The mutant-Kirsten rat sarcoma (KRAS)-targeting vaccine was reported to lower the risk of death by 77% and the risk of relapse by 88% in patients with colorectal cancer and pancreatic ductal adenocarcinoma (PDAC) in the Phase I AMPLIFY-201 trail (NCT04853017).
𝗥𝗶𝘀𝗶𝗻𝗴 𝗗𝗲𝗺𝗮𝗻𝗱 𝗳𝗼𝗿 𝗣𝗿𝗲𝗰𝗶𝘀𝗶𝗼𝗻 𝗜𝗺𝗺𝘂𝗻𝗼𝘁𝗵𝗲𝗿𝗮𝗽𝗶𝗲𝘀 𝗥𝗲𝘀𝗵𝗮𝗽𝗲𝘀 𝗠𝗮𝗿𝗸𝗲𝘁 𝗗𝘆𝗻𝗮𝗺𝗶𝗰𝘀
The market’s expansion is being shaped by a combination of clinical necessity and scientific progress. Globally, the oncology landscape is witnessing a growing emphasis on immune-based interventions that can complement or enhance existing treatment protocols such as chemotherapy, radiotherapy, and immune checkpoint inhibitors.
Key forces influencing adoption include:
• Increasing global cancer incidence and recurrence rates
• Limitations of one-size-fits-all oncology treatments
• Growing acceptance of personalized medicine in clinical practice
• Expanding number of peptide vaccine candidates in clinical pipelines
• Strong academic industry collaboration in cancer immunology
Healthcare providers are increasingly exploring peptide vaccines as maintenance or adjuvant therapies, particularly in cases where long-term immune surveillance is essential.
𝗦𝗰𝗶𝗲𝗻𝘁𝗶𝗳𝗶𝗰 𝗔𝗱𝘃𝗮𝗻𝘁𝗮𝗴𝗲 𝗼𝗳 𝗣𝗲𝗽𝘁𝗶𝗱𝗲 𝗖𝗮𝗻𝗰𝗲𝗿 𝗩𝗮𝗰𝗰𝗶𝗻𝗲𝘀
Peptide-based vaccines offer several distinct benefits that set them apart within the immunotherapy space:
• High specificity toward tumor-associated antigens
• Lower toxicity compared to cell-based or viral vaccines
• Ease of manufacturing and scalability
• Compatibility with combination therapies
• Improved safety profiles for long-term administration
These characteristics make peptide vaccines particularly attractive for chronic cancer management, post-remission treatment, and early-stage intervention strategies.
💠𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐚𝐩𝐩𝐥𝐢𝐜𝐚𝐭𝐢𝐨𝐧-𝐥𝐞𝐯𝐞𝐥 𝐢𝐧𝐬𝐢𝐠𝐡𝐭𝐬: https://www.intelmarketresearch.com/global-india-softwood-lumber-forecast-market-10310
𝐈𝐧𝐝𝐮𝐬𝐭𝐫𝐲 𝐒𝐞𝐠𝐦𝐞𝐧𝐭 𝐀𝐧𝐚𝐥𝐲𝐬𝐢𝐬
▪️𝗕𝘆 𝗧𝘆𝗽𝗲
• ITK-1
• GRN-1201
• TPIV200
• TPIV110
• UV1
• Galinpepimut-S
• TARP 27-35
• HER-Vaxx
• Vx-001
• Others
UV1 is expected to dominate the target market growth because it targets a universal cancer antigen (telomerase/hTERT) that is expressed in the vast majority of solid tumors, enabling broad clinical applicability across multiple cancer types while maintaining a strong safety and immune-response profile.
▪️𝗕𝘆 𝗔𝗽𝗽𝗹𝗶𝗰𝗮𝘁𝗶𝗼𝗻
• Breast Cancer
• Lung Cancer
• Melanoma
• Prostate Cancer
• Others
Melanoma is one of the most immunogenic tumors, which means it elicits a robust and quantifiable immune response, making it ideal for peptide-based vaccine development and clinical validation. This is the main reason why the melanoma segment dominates the peptide cancer vaccine market.
💠𝗖𝗹𝗶𝗰𝗸 𝘁𝗵𝗶𝘀 𝗹𝗶𝗻𝗸 𝘁𝗼 𝗱𝗼𝘄𝗻𝗹𝗼𝗮𝗱 𝘁𝗵𝗲 𝘀𝗮𝗺𝗽𝗹𝗲 𝗿𝗲𝗽𝗼𝗿𝘁: https://www.24lifesciences.com/download-sample/7842/peptide-cancer-vaccine-market
▪️𝗕𝘆 𝗘𝗻𝗱 𝗨𝘀𝗲𝗿
• Hospitals
• Specialty Oncology Clinics
• Academic & Research Institutes
The majority of peptide cancer vaccines are still in the discovery, preclinical, and early clinical trial stages, where universities and research institutions play a crucial role, which is why the Academic & Research institutions sector dominates the peptide cancer vaccine market.
▪️𝗕𝘆 𝗗𝗲𝘃𝗲𝗹𝗼𝗽𝗺𝗲𝗻𝘁 𝗣𝗵𝗮𝘀𝗲
• Preclinical
• Phase I/II Clinical Trials
• Phase III Clinical Trials
Since most peptide-based cancer vaccines are still in early to mid-stage development, where safety validation, immune response profiling, optimal antigen selection, and dosing strategies are established prior to large-scale efficacy trials, the Phase I/II clinical trials segment dominates the peptide cancer vaccine market.
▪️𝗕𝘆 𝗧𝗵𝗲𝗿𝗮𝗽𝗲𝘂𝘁𝗶𝗰 𝗔𝗽𝗽𝗿𝗼𝗮𝗰𝗵
• Monotherapy
• Combination Therapy
• Adjuvant Therapy
The market for peptide cancer vaccines is dominated by combination therapy since it significantly boosts the effectiveness of the immune response when peptide vaccines are used in conjunction with immune checkpoint inhibitors, chemotherapy, or targeted therapies.
𝐂𝐨𝐦𝐩𝐞𝐭𝐢𝐭𝐢𝐯𝐞 𝐋𝐚𝐧𝐝𝐬𝐜𝐚𝐩𝐞 𝐚𝐧𝐝 𝐈𝐧𝐝𝐮𝐬𝐭𝐫𝐲 𝐏𝐚𝐫𝐭𝐢𝐜𝐢𝐩𝐚𝐭𝐢𝐨𝐧
The peptide cancer vaccine ecosystem is characterized by a mix of biotechnology innovators, pharmaceutical leaders, and research-driven start-ups. Market participants are focusing on:
• Expanding clinical trial portfolios
• Developing next-generation peptide formulations
• Exploring synergistic combinations with immune checkpoint inhibitors
• Leveraging genomic data for antigen selection
Strategic partnerships between biotech firms and academic institutions are accelerating innovation while reducing development risk.
🔸Boston Biomedical
🔸Ultimovacs
🔸BrightPath Biotherapeutics
🔸TapImmune (acquired by Marker Therapeutics)
🔸Immatics
🔸Sellas Life Sciences
🔸Imugene
🔸VAXON Biotech
🔸Generex Biotechnology
🔸ISA Pharmaceuticals
🔸OncoTherapy Science
🔸Eli Lilly and Company (through acquisition)
🔸GSK
🔸CureVac
🔸BioNTech SE
💠𝐕𝐢𝐞𝐰 𝐭𝐡𝐞 𝐜𝐨𝐦𝐩𝐥𝐞𝐭𝐞 𝐦𝐚𝐫𝐤𝐞𝐭 𝐚𝐧𝐚𝐥𝐲𝐬𝐢𝐬 𝐛𝐲 𝐜𝐥𝐢𝐜𝐤𝐢𝐧𝐠 𝐭𝐡𝐢𝐬 𝐥𝐢𝐧𝐤: https://www.24lifesciences.com/peptide-cancer-vaccine-market-7842
𝗥𝗲𝗴𝗶𝗼𝗻𝗮𝗹 𝗢𝘂𝘁𝗹𝗼𝗼𝗸 𝗛𝗶𝗴𝗵𝗹𝗶𝗴𝗵𝘁𝘀
◘ 𝐍𝐨𝐫𝐭𝐡 𝐀𝐦𝐞𝐫𝐢𝐜𝐚
North America remains a leading region due to strong oncology research funding, advanced clinical trial infrastructure, and early adoption of immunotherapies. The U.S. continues to be a hub for peptide vaccine development and regulatory progress.
◘ 𝐄𝐮𝐫𝐨𝐩𝐞
Europe shows steady growth supported by collaborative cancer research programs and favorable regulatory frameworks for advanced therapies. Several EU countries are investing in personalized oncology initiatives.
◘ 𝐀𝐬𝐢𝐚-𝐏𝐚𝐜𝐢𝐟𝐢𝐜
Asia-Pacific is emerging as a high-growth region, driven by increasing cancer prevalence, improving healthcare infrastructure, and rising clinical research activity in countries such as China, Japan, and South Korea.
◘ 𝐑𝐞𝐬𝐭 𝐨𝐟 𝐭𝐡𝐞 𝐖𝐨𝐫𝐥𝐝
Other regions are gradually expanding participation through international clinical trials, public health investments, and technology transfer initiatives.
𝗘𝗺𝗲𝗿𝗴𝗶𝗻𝗴 𝗧𝗿𝗲𝗻𝗱𝘀 𝗦𝗵𝗮𝗽𝗶𝗻𝗴 𝘁𝗵𝗲 𝗠𝗮𝗿𝗸𝗲𝘁
• Integration of peptide vaccines with immune checkpoint inhibitors
• Use of AI-driven antigen prediction tools
• Growth of neoantigen discovery platforms
• Expansion of therapeutic cancer vaccines beyond late-stage disease
• Increased focus on preventive cancer immunotherapy research
• Improved peptide delivery systems and adjuvants
These developments highlight the market’s transition from experimental research toward clinically relevant and commercially viable solutions.
𝐎𝐥𝐝 𝐜𝐨𝐧𝐜𝐞𝐩𝐭𝐬, 𝐧𝐞𝐰 𝐦𝐞𝐭𝐡𝐨𝐝𝐬: 𝐇𝐨𝐰 𝐚𝐫𝐞 𝐩𝐞𝐩𝐭𝐢𝐝𝐞 𝐯𝐚𝐜𝐜𝐢𝐧𝐞𝐬 𝐜𝐡𝐚𝐧𝐠𝐢𝐧𝐠 𝐜𝐚𝐧𝐜𝐞𝐫 𝐢𝐦𝐦𝐮𝐧𝐨𝐭𝐡𝐞𝐫𝐚𝐩𝐲?
Over the past few decades, research on cancer immunotherapy has firmly established immune cells as key players in effective cancer treatment. Peptide vaccines directly targeting immune cells have demonstrated immense potential due to their specificity and applicability. However, developing peptide vaccines to generate tumor-reactive T cells remains challenging, primarily due to suboptimal immunogenicity and overcoming the immunosuppressive tumor microenvironment (TME).
𝐃𝐨 𝐈𝐧𝐯𝐞𝐬𝐭𝐨𝐫𝐬 𝐂𝐚𝐫𝐞 𝐀𝐛𝐨𝐮𝐭 𝐓𝐡𝐢𝐬 𝐌𝐚𝐫𝐤𝐞𝐭? 𝐒𝐞𝐞 𝐇𝐨𝐰?
Organizations operating across oncology, biotechnology, and pharmaceutical development are increasingly viewing peptide cancer vaccines as a strategic long-term opportunity. Their modular design, scalability, and compatibility with precision medicine frameworks make them well-suited for future cancer treatment models.
Important Takeaways from the Complete Report:
1. Market size forecasts through 2032
2. Pipeline analysis of peptide vaccine candidates
3. Competitive benchmarking and strategic developments
4. Regional investment and clinical trial trends
5. Technology advancements shaping next-generation vaccines
6. Strategic recommendations for market entry and expansion
💠𝗖𝗹𝗶𝗰𝗸 𝘁𝗵𝗶𝘀 𝗹𝗶𝗻𝗸 𝘁𝗼 𝗱𝗼𝘄𝗻𝗹𝗼𝗮𝗱 𝘁𝗵𝗲 𝘀𝗮𝗺𝗽𝗹𝗲 𝗿𝗲𝗽𝗼𝗿𝘁: https://www.24lifesciences.com/download-sample/7842/peptide-cancer-vaccine-market
💠𝗙𝗲𝗲𝗹 𝗙𝗿𝗲𝗲 𝘁𝗼 𝗥𝗲𝗮𝗰𝗵 𝗢𝘂𝗿 𝗠𝗼𝘀𝘁 𝗥𝗲𝗰𝗲𝗻𝘁 𝗨𝗽𝗱𝗮𝘁𝗲𝘀 𝗼𝗳 𝘁𝗵𝗲 𝗖𝗼𝗺𝗽𝗹𝗲𝘁𝗲 𝗥𝗲𝗽𝗼𝗿𝘁: https://www.24lifesciences.com/peptide-cancer-vaccine-market-7842
𝗣𝗼𝘁𝗲𝗻𝘁𝗶𝗮𝗹 𝗳𝗼𝗿 𝘁𝗵𝗲 𝗙𝘂𝘁𝘂𝗿𝗲 𝗼𝗳 𝗣𝗲𝗽𝘁𝗶𝗱𝗲 𝗖𝗮𝗻𝗰𝗲𝗿 𝗩𝗮𝗰𝗰𝗶𝗻𝗲𝘀
Peptide cancer vaccine market is entering a decisive phase marked by scientific validation, regulatory progress, and growing clinical confidence. As oncology shifts toward immune-based and patient-specific solutions, peptide vaccines are expected to play a complementary yet increasingly impactful role.
Ongoing research into antigen discovery, delivery optimization, and combination therapies will continue to refine efficacy outcomes. Over the next decade, peptide cancer vaccines are likely to move beyond experimental settings and become an integral component of personalized cancer care pathways worldwide.
𝐑𝐞𝐥𝐚𝐭𝐞𝐝 𝐑𝐞𝐩𝐨𝐫𝐭𝐬:
➜ 𝗗𝗲𝗻𝗱𝗿𝗶𝘁𝗶𝗰 𝗖𝗲𝗹𝗹 𝗖𝗮𝗻𝗰𝗲𝗿 𝗩𝗮𝗰𝗰𝗶𝗻𝗲𝘀 𝗠𝗮𝗿𝗸𝗲𝘁: https://www.24lifesciences.com/dendritic-cell-cancer-vaccines-market-9270
➜ 𝗺𝗥𝗡𝗔 𝗖𝗮𝗻𝗰𝗲𝗿 𝗩𝗮𝗰𝗰𝗶𝗻𝗲𝘀 𝗮𝗻𝗱 𝗧𝗵𝗲𝗿𝗮𝗽𝗲𝘂𝘁𝗶𝗰𝘀 𝗠𝗮𝗿𝗸𝗲𝘁: https://www.24lifesciences.com/mrna-cancer-vaccines-and-therapeutics-market-6771
➜ 𝗦𝘆𝗻𝘁𝗵𝗲𝘁𝗶𝗰 𝗣𝗲𝗽𝘁𝗶𝗱𝗲 𝗩𝗮𝗰𝗰𝗶𝗻𝗲𝘀 𝗳𝗼𝗿 𝗩𝗲𝘁𝗲𝗿𝗶𝗻𝗮𝗿𝘆 𝗨𝘀𝗲 𝗠𝗮𝗿𝗸𝗲𝘁: https://www.24lifesciences.com/synthetic-peptide-vaccines-for-veterinary-use-market-market-5296
➜ 𝗕𝗮𝗰𝘁𝗲𝗿𝗶𝗮𝗹 𝗩𝗮𝗰𝗰𝗶𝗻𝗲𝘀 𝗠𝗮𝗿𝗸𝗲𝘁: https://www.24lifesciences.com/bacterial-vaccines-market-9056
➜ 𝗙𝗹𝘂 𝗥𝗡𝗔 𝗩𝗮𝗰𝗰𝗶𝗻𝗲𝘀 𝗠𝗮𝗿𝗸𝗲𝘁: https://www.24lifesciences.com/flu-rna-vaccines-market-market-5741
𝐀𝐛𝐨𝐮𝐭 𝟐𝟒𝐥𝐢𝐟𝐞𝐬𝐜𝐢𝐞𝐧𝐜𝐞𝐬
Founded in 2017, 24LifeScience has emerged as a trusted research and analytics partner for organizations operating within the global life sciences and chemical industries. Our core mission is to provide intelligent, future-ready insights that help clients stay ahead in an increasingly complex and innovation-driven market.
𝐈𝐧𝐭𝐞𝐫𝐧𝐚𝐭𝐢𝐨𝐧𝐚𝐥: +91 9425150513 (Asia)
𝐖𝐞𝐛𝐬𝐢𝐭𝐞: http://www.24lifesciences.com
𝐅𝐨𝐥𝐥𝐨𝐰 𝐮𝐬 𝐨𝐧 𝐋𝐢𝐧𝐤𝐞𝐝𝐈𝐧: http://www.linkedin.com/company/lifesciences24
Darsh
24lifesciences
+91 9425150513
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.